Dyad Labs Protein Testing for USP Dietary Proteins Expert Panel
resources - Recent Conference Materials
Dyad Labs Protein Testing for USP Dietary Proteins Expert Panel
Sample preparation
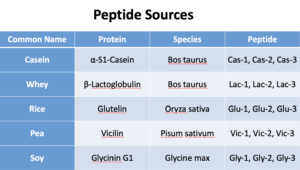
For dietary proteins, our sample preparation is top of the line. We weigh the sample, then we add 5.0M urea in 50 mM Tris buffer. After that, we reduce the sample with 1 M dithiothreitol (DTT) for 30 minutes, and add Alkylate in 0.5 M iodoacetamide for 30 minutes. The sample is then digested with trypsin solution at 37°C overnight. The sample is then quenched with formic acid. We filter it with a disposable syringe and 0.22 μm nylon syringe filter. Finally, it is diluted (1:10) with 0.1% formic acid and 200 ng/mL internal standard, β-casomorphin 1-4 peptide.
Chromatography
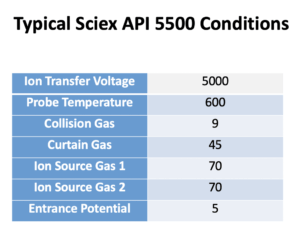
We use the Shimadzu Nexera system. In Mobile phase A we use 0.1% formic acid in water. In Mobile phase B we use 0.1% formic acid in acetonitrile. The Flow Rate is 0.3 mL/min. Column: C18, 150 × 2 mm, Synergi 4 μ Hydro 80 Å (P/N 00F-4375-B0; Phenomenex). See charts below for more info.
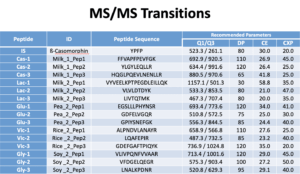
Data processing
Raw materials identified using PCR are used to set the Method Reporting Limit (MRL). If all three peptides from same protein have larger peak area than the MRL (1000 ppm), a positive identification is made.
aoac method
The Protein identification method received AOAC First Action status in December 2017. AOAC Official Method 2017.11: Identification of Pea, Rice, and Soy Proteins in Raw Materials and Finished Goods. AOAC Official Method 2017.12: Identification of Milk Proteins in Raw Materials and Finished Goods.
validation- selectivity, poi
Let’s review a few concepts regarding validation.
- Negative Selectivity: Two different matrices, six replicates each, no analyte peaks detected
- Positive Selectivity: Matrix with analyte only for each protein, no other analyte peaks detected, other than the analyte of interest
- Probability of Identification at low concentration: 18 different matrices with MRL at 100 ppm for each (except rice at 500 ppm); all confirmed presence, except rice was 17/18 confirmed.
- Probability of Identification at high concentration: Two different matrices, six replicates each all confirmed presence of analyte at 10,000 ppm.
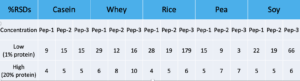
When it comes to precision, the Low and High concentrated sample was prepared six times. %RSDs for each peptide shown below:
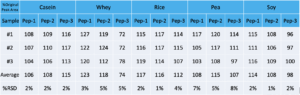
Regarding reinjection reproducibility, samples were stored at 1-8C for 5 days and then reinjected. The following are % peak areas compared to original.
The following compounds were analyzed in blank matrix and no analyte peaks were found: Creatine, Beta alanine, Histidine, Taurine, Serine, Citrulline, Caffeine, Phenylalanine, Tryptophan, Metaline, Urea, Methionine, Isoleucine, Arginine, Glycine, Lysine, Leucine, Valine, Threamine, and Tyrosine.
Testing USP samples
Dyad tested the following samples on October 23, 2018.
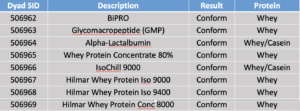
In round 2, Dyad tested fish gelatin samples on March 7, 2019.
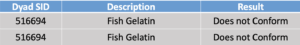
Some peptides had peaks: Gly-3 (~800% of MRL), Cas-1 (~0.5% of MRL), and Cas-2 (~1% of MRL). Dyad believes that the method is suitable to put into USP whey monograph.
Some important considerations
LC/MS/MS is becoming a more common instrument in labs that test dietary supplements and foods. The estimated cost for analysis, including labor, instrumentation, reagents, consumables are: $197/first sample, $31/each additional sample.
Moving forward, our next steps will be to consider if the peaks in the fish gelatin samples peptides are from fish gelatin protein or something else? Does this matter? We will also consider different protein sources that can be added to the method as needed.