Quantifying 16 Cannabinoids in Hemp Flower and Leaf using LC/MS/MS
resources - Recent Conference Materials
A Fast, Sensitive and Comprehensive Assay to Quantify 16 Cannabinoids in Hemp Flower and Leaf using LC/MS/MS
Introduction
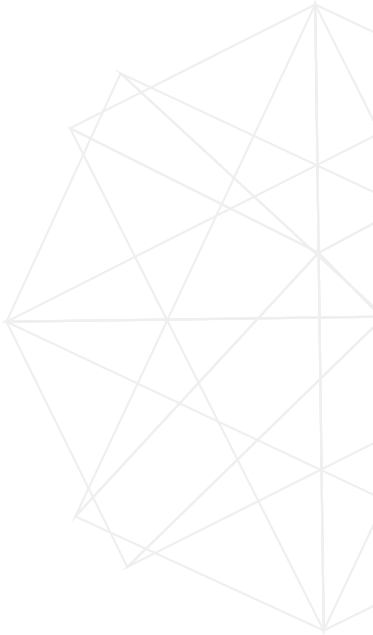
Marijuana is defined as any cannabis sativa plant that has greater than 0.3 percent tetrahydrocannabinol (THC), while hemp is a phenotype of the Cannabis sativa plant species that has 0.3 percent or less THC. In 2018, AOAC published a method to quantify 10 cannabinoids (CBDs) with the range of 0.500-10.0 μg/mL in finished goods. However, there are more than 100 CBDs isolated from cannabis in addition to these 10 CBDs in the AOAC method. In 2019, Dyad Labs validated a LC/MS/MS method for 16 CBDs in distillate, extract, and other finished products. In this paper, Dyad Labs has successfully developed and validated another fast, sensitive, and comprehensive LC/MS/MS assay to quantify 16 cannabinoids in the hemp leaf and flower samples.
Methodology
The hemp sample was homogenized to powder by Resch Mixer Mill MM400. 0.500 gram of hemp powder was weighed and extracted with ethanol: water (90:10, v/v) in an amber container, followed by filtration with 0.45 μm membrane filter. The internal standards, including cannabidiol-d3, cannabinol-d3, delta9-THC-d3, were used in this method. Extracts were injected for analysis on Shimadzu Nexera UPLC system with an ARC-18 column at 35 °C.
Results and Methods
Stability
In the AOAC method, the standard solutions are expensive and only have 3 days of stability. In order to better understand the stability of CBD, we comprehensively investigated on the stability of all CBDs with different parameters including light, heat and pH. The study indicated that CBDs are sensitive to light, heat and pH. Neutral condition with light protection and low temperature was adopted during sample preparation. Dyad Labs has successfully established stability up to 149 days.
Chromatographic Separation
Some CBDs are isomers and have the same MRM transitions, thus chromatographic separation is very critical in this method. We have investigated on different columns, mobile phases and buffers to achieve enough separation, sharp and symmetric peak shape. A special C18 column provided sufficient separation.
Accuracy and Precision
The accuracy and precision were investigated with post-spiking all sixteen CBDs in hemp leaf and flower at lower, medium, and high regions of the established range of the calibration curve (20.0-2000 mg/mL).
Conclusion
This method is a fast, sensitive, comprehensive and accurate method and is the first validated method to simultaneously quantify 16 major CBDs in hemp flower and leaf samples using UPLC‐MS/MS.