Protein Testing – Nowhere to Hide
resources - Recent Conference Materials
An update on Genysis Labs ‘Protein Verified’ Technology
Nowhere to hide
In the last industry meeting, Dr. Spencer Carter outlined a new LC/MS/MS method of protein testing. Today, we are excited to share with you an update on this technology. But first… a quick review with 4 key questions:
- Where is the industry today?
- What are the specific challenges?
- Why does it matter?
- How are DNA and LC/MS/MS different?
the state of protein supplements
Where is the industry today? Well, this is the $4.7 B Question. Protein represents 70% of the total revenue for the Sports Nutrition category. The most widely utilized protein test methods do not adequately screen for adulteration. Customers are demanding more detailed information and transparency. But as always, the FDA is listening and watching what is happening in the industry.
“A growing number of companies are accused of selling workout supplements spiked with cheap fillers that they’re passing off as protein.”
— Forbes Magazine 2015
Jennifer Dooren also said, “FDA requires that dietary supplements be labeled in a manner that is truthful and not misleading. With regard to the labeling of protein content, FDA’s expectation for proper nutrition labeling is that firms will evaluate the protein content from actual protein sources—not other nitrogen-containing ingredients such as individual amino acids—and label the products consistent with the results of such evaluations.”
Three dimensions of protein testing
The three dimensions of protein testing are identification, adulteration, and quantification. Let’s take a look at each of these pieces more closely.
Identification: This dimension investigates which proteins are present. Protein comes from a variety of animal and plant sources. The ideal tests allow us to identify specific proteins in each sample such as whey, pea, rice, casein, soy and many more.
Adulteration: It’s important to investigate if there’s more inside your protein than you asked for. Let’s look at the example of a pea protein containing a small amount of soy. DNA testing confirms the presence of pea protein. Additional DNA screening of other compounds, including soy, were tested. If no soy was detected, other adulterants could include free amino acids or melamine.
Quantitation: The sample is then tested with additional screening.
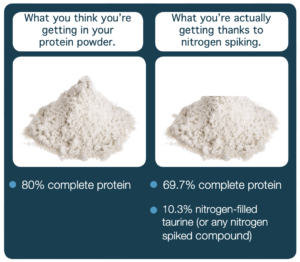
What you think you’re getting in your protein powder is not always what you’re actually getting. Nitrogen spiking affects your protein, but current Kjeldahl tests are not selective for the nitrogen source. A free amino acid test is selective for each amino acid. Without testing, you may only be getting 60% complete protein.
The difference between DNA and LC/MS/MS
This is very innovative and new to our industry. Using LC/MS/MS technology for Identification (and soon for Adulteration and Quantitation) is not simple stuff. It allows us to find unique peptides for each protein. We use recombinant DNA to create new standards. It’s like Jurassic Park- they extracted the dinosaur DNA from mosquito fossils to incubate new dinosaurs. Our new method is protein verified. We extract DNA from the target proteins to incubate protein standards.
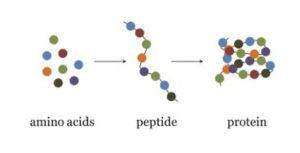
The process of Protein Identification is very thorough and complex. First we identify specific protein of interest. We break down protein into smaller peptide fragments using tryptic digest. Then we select three unique peptides specific to the protein of interest.
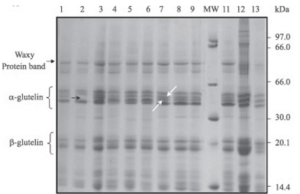
We analyze with Liquid Chromatography with tandem Mass Spectrometry (LC/MS/MS). Multiple proteins are present in samples. We select a specific protein based on literature data. The example on the right shows the SDS-Page of seed total protein in rice. Gluten is chosen here.
Sample Preparation
The Trypsin enzyme hydrolyzes proteins. Cleavage occurs mainly at the carboxyl side of the amino acids Lysine (K) and Arginine (R). Smaller tryptic peptide fragments result from cleavage. Take a look at this Pea vs. Soy peptide selection example below.
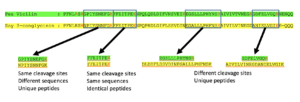
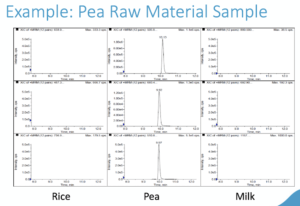
LC/MS/MS Protein Identification
The LC/MS/MS Protein Identification process occurs when peptides separated from each other and the components in the extract by liquid chromatography. Peptides can be detected in a mass spectrometer based on their mass-to-charge (m/z) ratio in positive-ion mode. Take a look at the Pea Raw Material Sample below to see an example of this in action. The LC/MS/MS Protein ID Method has been validated with the following tests: Method Reporting Limit, Selectivity, Specificity, Precision, and Reinjection Reproducibility.